Governance
The Answer ALS Neuromine Platform operates under comprehensive governance policies. These policies delineate Answer ALS Neuromine Platform users’ rights and responsibilities as well as reference rights and responsibilities of Answer ALS governing bodies.
The following sections specify the Answer ALS Neuromine Platform Terms and Conditions of Use. These establish the use, disclaimers, and limitation of liability governing the use of the Answer ALS Neuromine Platform. By using the Answer ALS Neuromine Platform, you are agreeing to abide by each of these terms and conditions.
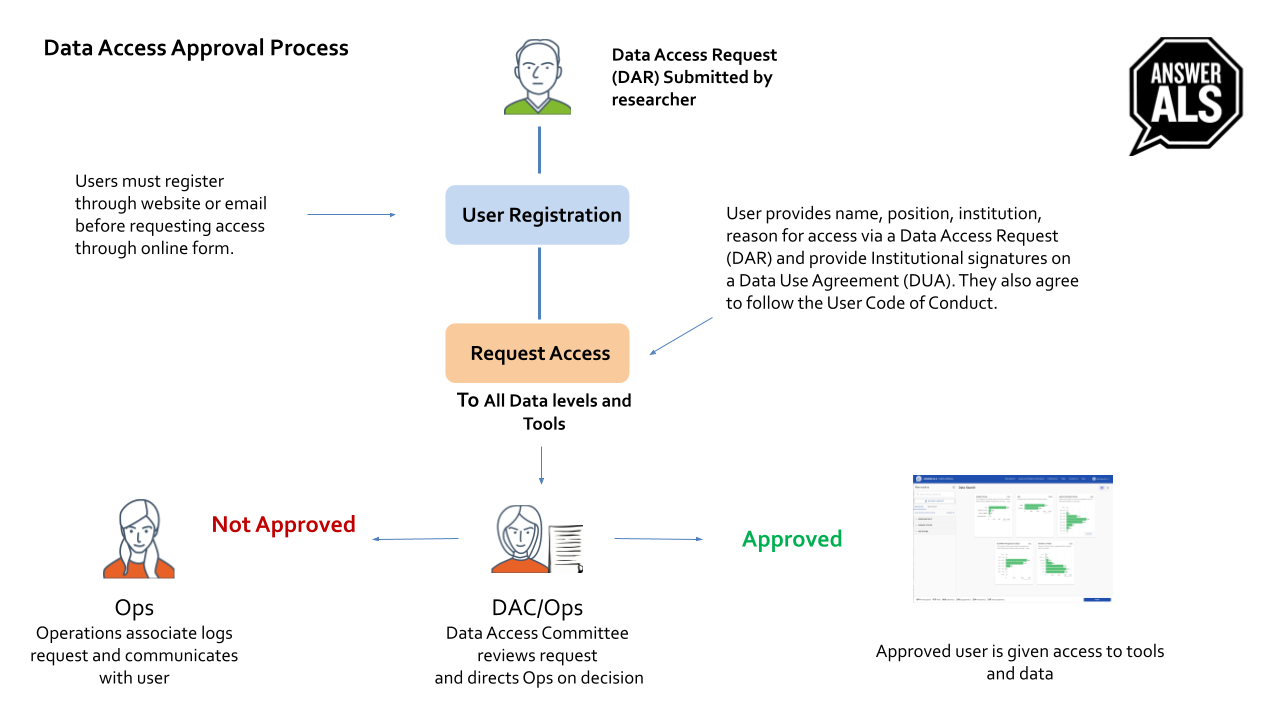
Answer ALS Platform users will have the opportunity to register with Answer ALS. This registration allows the users to receive updates from Answer ALS about upcoming data releases, new tools and upcoming events.
- Public Access – Browse the site for updates, information and links to repositories and OMIC centers.
- Controlled Data Access – Full dataset access to the individual-level de-identified clinical and omics data and metadata will be granted through the completion of an online registration form, signature on the Data Use Agreement with the individual requestor’s Authorized Institutional Business Official signature and verification of the email address.
Once approved for access, Answer ALS Platform users will have access to the following:
- Answer ALS Cohort data.
- Answer ALS Neuromine Data Explorer to visualize data and create cohorts.
- Download de-identified clinical data, Levels 1-4 epigenomic, proteomic, transcriptomic, and genomic data.
- Access to visualization tools such as heatmap, PCA, boxplots, and more to explore selected cohort data.
- Order cell lines and biosamples collected from a selected Answer ALS cohort.
Answer ALS uses DocuSign for requestor, institutional and collaborator signatures and a web-based, semi-automated Data Sharing Application and Tracking system to manage the data requests. Users will complete a web-based application form and sign a Data Use Agreement via DocuSign. When the application is received, the prospective user will notified that their request submission was successful. Once the request is approved, the applicant will be given permission to access the data through the Answer ALS Neuromine Platform.
Applicants will be required to provide the following information. Please note that this information should be collected prior to requesting access. This will help your data access process to proceed.:
- First and Last Name
- Credentials (eg. PhD, MD, Director)
- Email address
- Academic Affiliation (if any) or Institution/Company Name
- Principal Investigator for the project. This is very important if the requestor is a graduate student, postdoc, scientist, or research associate. You must have your supervising PI, Director, or above co-sign the DUA.
- A Business Official also needs to sign on behalf of your associated entity. Please contact your contracts office or PI to identify the correct person for this role. Answer ALS does not know the policies of each Institution and therefore suggests you ensure the right person is identified for this role. It is entirely up to the data requestor to determine who should sign for the University, Institution, or Company.
- Proposed analyses.
- Project Timeframe.
- Data types and levels you are interested in (Epigenomics, Proteomics, Transcriptomics, Genomics; levels 1-4).
- Names and emails of collaborators using the data
- Certification to each point of the Data Use Agreement
- Read and agree to a Data User Code of Conduct
- Read and agree to Answer ALS Consent to Public Use
Applicants might receive requests to update their registration information.
A data access committee (DAC) plays a critical role in ensuring the responsible and ethical use of sensitive data, particularly in scientific research and clinical trials. The composition of such a committee is designed to reflect the different perspectives and interests involved in the data access process.
- Scientific representative: This person is usually a researcher or scientist who is knowledgeable about the scientific and technical aspects of the data being requested. They can provide important insights into the feasibility and potential impact of the proposed research.
- Patient and patient advocates: These individuals represent the interests of the patients whose data is being requested. They play a crucial role in ensuring that the privacy and confidentiality of patients is protected, and that their rights are respected.
- Program director: The program director is responsible for overseeing the administration and implementation of the data access process. They are responsible for ensuring that the committee follows established protocols and policies, and that the decisions made by the committee are consistent and fair.
- External data trust reviewer: This person is an independent expert who specializes in data privacy and security. They are responsible for evaluating the security and privacy of the data being requested, and ensuring that appropriate safeguards are in place to protect sensitive information.
- Rotating external member: This person is selected from a pool of experts in relevant fields, such as ethics, law, or medicine. They provide a fresh perspective on each data access request, and help to ensure that the committee is making decisions based on the latest best practices and current knowledge.
In conclusion, the composition of a data access committee reflects the importance of balancing the needs of science, patients, and data protection. The diverse perspectives represented on the committee ensure that decisions are made in a transparent, responsible, and ethical manner.
To create the best platform possible for sharing and collaborating in research, including best practices for data security, users who want to download Answer ALS data to local servers must provide an institutional signature verifying that their host institution adopts responsibility for the security of the downloaded data. This is incorporated into the DUA. In addition, Answer ALS will require a data use proposal to understand how each requestor intends to use the data.
To download or transfer data, users need to download the Answer ALS Download tool to their computer and follow the instructions provided. Once registered with the site, you can navigate to the user menu to download the tool.
Answer ALS is excited to hear about your manuscripts and publications using the data provided. Below are guidelines for acknowledging and providing Answer ALS with copies of your manuscripts.
ANSWER ALS Acknowledgement:
“Data used in the preparation of this article were obtained from the ANSWER ALS Data Portal (AALS-01184). For up-to-date information on the study, visit https://dataportal.answerals.org or https://www.answerals.org.
ANSWER ALS Cohort Acknowledgements:
“Clinical data and biosamples used in the preparation of this article were obtained from the (i) Answer ALS Foundation Program ‘Answer ALS’. For up-to-date information on the program, visit https://www.answerals.org.
In addition, as stated in the DUA, researchers will provide either (i) a copy of the manuscript upon its acceptance for publication or (ii) the full citation of all published manuscripts to the ANSWER ALS Publications Committee. Citations will be listed on the ANSWER ALS websites and available to the public through PubMed.
To view a list of known publications using Answer ALS data, please click here.
Answer ALS (AALS) is a project dedicated to developing and implementing a unified strategy to identify new therapeutic targets for Amyotrophic Lateral Sclerosis (ALS). We achieve change by unifying our community toward agreed-upon goals in research, science, technology, and education. Implicit in establishing the Answer ALS data and cell repositories is the view that scientific progress in this area will be significantly enhanced if the data produced by these studies are readily available to all investigators in the research community.